- Joined
- 19 June 2012
- Posts
- 28
- Reactions
- 0
..bottom wedge is forming... indicates that the price may rise to the range of A$1.15/sh to A$1.40/sh.
Current price 98c.
Current price 98c.
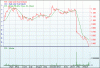
Australian drug maker Pharmaxis faces an uncertain future after one of its key drugs, Bronchitol, failed to reach the targets needed for it to be submitted for regulatory approval.
Shares in Pharmaxis plunged 52.4 per cent to 15 ¢ on Wednesday after it said its year-long phase three study of 485 patients with bronchiectasis, a lung disease, recorded a statistically insignificant fall in exacerbation rates.
Two months ago the same drug was rejected by the main US regulatory body, the Food and Drug Administration, for marketing to cystic fibrosis patients.
Pharmaxis stocks are now down 87.9 per cent in 2013, after a 45.6 per cent fall in trading on January 31 when advisers to the FDA negatively reviewed the drug.
"There's a lot of uncertainty," BBY healthcare and life sciences analyst Dennis Hulme said.
"Now that the [bronchiectasis] potential is off the table, it looks like Pharmaxis will only have its modest sales in Europe and Australia for the next three years. So they would need to slash their costs substantially to survive in that model."
Pharmaxis chief executive Gary Phillips said the results of the trial were disappointing but added clinicians involved in the trial felt the results were ''highly clinically relevant''. ''Clearly there's a disconnect here in what would satisfy a regulator in terms of going for an approval for the drug, and what clinicians would take as convincing evidence to want to use it in their patients,'' he said.
Mr Phillips said Pharmaxis' business model he had been working on had not assumed the bronchiectasis trial would produce a positive result, and that the company would focus on growing its revenue from the use of Bronchitol in countries where the drug has been approved.
Pharmaxis had said this month that it was working with the FDA to design a new Bronchitol trial to improve its chances of breaking into the US market.
Biotechs need a lot of projects happening at once, don't they? otherwise the risk is too high.

well, here we are..On December 8th, 2023, Pharmaxis Ltd (PXS) changed its name and ASX code to Syntara Limited (SNT).



Hello and welcome to Aussie Stock Forums!
To gain full access you must register. Registration is free and takes only a few seconds to complete.
Already a member? Log in here.