- Joined
- 23 March 2005
- Posts
- 1,943
- Reactions
- 1
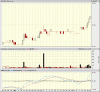
Major US Distributor announced!
I'm on 20 min delay, so I don't know details, but might be good for a quick trade for you guys
I'm on 20 min delay, so I don't know details, but might be good for a quick trade for you guys