- Joined
- 31 August 2005
- Posts
- 189
- Reactions
- 1
About Apollo Life Sciences Limited
Apollo Life Sciences (ASX:AOP) is a biotechnology company that has made major breakthroughs in the areas of drug delivery and expression of proteins from human cells. Apollo’s combined technologies are expected to lead to more effective and lower cost therapeutics, compared to first generation protein-based drugs.
--------------------------------------
I noticed these guys a few months back when there was a mention of their diabetes efforts. Sounded cool but I thought I'd chill for a bit.
Anyway, today an announcement of significance came out so I decided it was time to start following the action.
--------------------------------------
Today's announcement is as follows:
This reads very positively and it would appear they stand a great chance of commercialising this and at a quick rate which is rather unusual when human trials and testing have to get through - particularly in the USA.
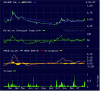
The chart shows good MACD for the last few days and volume is well and truly up. The all time high for the last 12 months is 85c. Today closed at 51c. I think this one looks pretty good at first glance but I've not done any of the fundamental checks.
It could pose a useful short trade.
Apollo Life Sciences (ASX:AOP) is a biotechnology company that has made major breakthroughs in the areas of drug delivery and expression of proteins from human cells. Apollo’s combined technologies are expected to lead to more effective and lower cost therapeutics, compared to first generation protein-based drugs.
--------------------------------------
I noticed these guys a few months back when there was a mention of their diabetes efforts. Sounded cool but I thought I'd chill for a bit.
Anyway, today an announcement of significance came out so I decided it was time to start following the action.
--------------------------------------
Today's announcement is as follows:
Apollo Life Sciences (ASX:AOP) announced today that the final report confirms the success of its oral insulin in Phase 1 toxicology trials. Apollo’s oral insulin consists of its oral delivery technology, Oradel™, loaded with generic insulin. The treatment proved safe at both low and high doses.
The toxicology study was designed to test the safety of Apollo’s oral insulin for people with diabetes. Oradel™ insulin was administered daily to two animal species for 14 days, followed by a 14-day recovery period. Control groups received treatment with the vehicle alone.
The key findings are that Oradel™ insulin caused no toxic effects or treatment-related changes in body weights, food consumption, blood analysis, urinalysis, gross necroscopy or histopathology.
“These favourable results provide strong supporting safety data for taking our oral insulin to Phase 1 human clinical trials, which we expect to undertake in the coming months,” said Dr Greg Russell-Jones, Apollo’s Science Director.
Diabetes is responsible for approximately 4 million deaths worldwide every year. It is estimated that there are now 246 million people with diabetes, growing to 380 million by 2025. A recent United Nations resolution calling on all countries to develop national policies for the prevention, treatment and care of diabetes acknowledges global concern at the prevalence of diabetes.
“Our innovative oral insulin could mean the end of needles for many people with diabetes. Some need up to five insulin shots a day to manage their condition,” said Dr Russell-Jones. “It is only a matter of time before we enter the marketplace with our insulin in a tablet, and we believe that most people prefer to take a tablet instead of an injection.”
The global market for diabetes medications is valued at $18 billion per annum.
--------------------------------------The toxicology study was designed to test the safety of Apollo’s oral insulin for people with diabetes. Oradel™ insulin was administered daily to two animal species for 14 days, followed by a 14-day recovery period. Control groups received treatment with the vehicle alone.
The key findings are that Oradel™ insulin caused no toxic effects or treatment-related changes in body weights, food consumption, blood analysis, urinalysis, gross necroscopy or histopathology.
“These favourable results provide strong supporting safety data for taking our oral insulin to Phase 1 human clinical trials, which we expect to undertake in the coming months,” said Dr Greg Russell-Jones, Apollo’s Science Director.
Diabetes is responsible for approximately 4 million deaths worldwide every year. It is estimated that there are now 246 million people with diabetes, growing to 380 million by 2025. A recent United Nations resolution calling on all countries to develop national policies for the prevention, treatment and care of diabetes acknowledges global concern at the prevalence of diabetes.
“Our innovative oral insulin could mean the end of needles for many people with diabetes. Some need up to five insulin shots a day to manage their condition,” said Dr Russell-Jones. “It is only a matter of time before we enter the marketplace with our insulin in a tablet, and we believe that most people prefer to take a tablet instead of an injection.”
The global market for diabetes medications is valued at $18 billion per annum.
This reads very positively and it would appear they stand a great chance of commercialising this and at a quick rate which is rather unusual when human trials and testing have to get through - particularly in the USA.
The chart shows good MACD for the last few days and volume is well and truly up. The all time high for the last 12 months is 85c. Today closed at 51c. I think this one looks pretty good at first glance but I've not done any of the fundamental checks.
It could pose a useful short trade.