- Joined
- 1 January 2007
- Posts
- 317
- Reactions
- 1
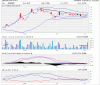
Yes the russian ministry has indicated that ropren will be approved in june (final approval as its already 99% approved anyway). they have pushed this date back a couple of times already but hopefully it will come through next week without any more delays.
Thanks Homer J - Well I'm sure this has already been taken into account with todays price if it's 99% done... But you never know..
Thanks