skc
Goldmember
- Joined
- 12 August 2008
- Posts
- 8,277
- Reactions
- 329
Re: Mesoblast bomb
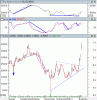
Feels a bit overdone for just a broker call... but they just responded to a speeding ticket with no answers...
MSB initiated at underperform by Macquarie.
Also big fade day today
Feels a bit overdone for just a broker call... but they just responded to a speeding ticket with no answers...