- Joined
- 27 June 2010
- Posts
- 4,147
- Reactions
- 309
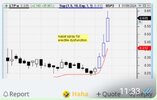
LTR Pharma Limited (LTP) is a drug development, research and repurposing company, focused on men’s health and, currently, commercialising a 'first-in-class' rapid onset, on demand therapeutic nasal spray for the treatment of ED.
LTP is currently focused on changing the method of administration of an existing and approved drug that is already on market for the treatment of ED called Vardenafil, also known by the brand names Levitra ® and Staxyn ®. LTP is preparing to launch SPONTAN®, which is based on an intranasal Vardenafil formulation, SDS-089, by undertaking a bioequivalence trial.
The Company plans to meet with, and make applications to, the FDA and TGA post its bioequivalence trial to seek an expedited approval process to sell and market SPONTAN® in the United States and in Australia.
The Company may also explore alternative avenues to make SPONTAN ® available to patients where necessary prior to it receiving regulatory approval from the TGA or FDA, for example SPONTAN® may be made available to patients via the TGA's special access schemes (SAS) or Authorised Prescriber Scheme (APS) on a needs basis and subject to the relevant regulatory framework.
 www.ltrpharma.com
www.ltrpharma.com
LTP is currently focused on changing the method of administration of an existing and approved drug that is already on market for the treatment of ED called Vardenafil, also known by the brand names Levitra ® and Staxyn ®. LTP is preparing to launch SPONTAN®, which is based on an intranasal Vardenafil formulation, SDS-089, by undertaking a bioequivalence trial.
The Company plans to meet with, and make applications to, the FDA and TGA post its bioequivalence trial to seek an expedited approval process to sell and market SPONTAN® in the United States and in Australia.
The Company may also explore alternative avenues to make SPONTAN ® available to patients where necessary prior to it receiving regulatory approval from the TGA or FDA, for example SPONTAN® may be made available to patients via the TGA's special access schemes (SAS) or Authorised Prescriber Scheme (APS) on a needs basis and subject to the relevant regulatory framework.
LTR Pharma – Clinical Stage Biopharmaceutical Company
 www.ltrpharma.com
www.ltrpharma.com