- Joined
- 3 May 2019
- Posts
- 6,182
- Reactions
- 9,669
After looking at ASX - CPH announcements, I found this which I consider to be broad scale impact news ;
26 November 2020
Update on TGA reclassification of CBD
▪ TGA decision regarding amendments to down schedule cannabidiol (CBD) products to schedule 3 medicine expected in late December 2020 (i)
▪ Schedule amendment would allow Australian consumers to purchase CBD products following consultation with a pharmacist without the need for a prescription
▪ Unlocks a large market opportunity for Creso Pharma – Australian market estimated to be worth upwards of A$200m (ii)
▪ Creso remains well funded to capitalise in Australian market following final decision.
For anyone who wonders why I think the ASX CPH announcement is relevant in the bigger picture and my opinion of why "it will be approved", I would encourage you to digest the information found here... (a wee bit of light reading)

4.1 Cannabidiol (private application) and cannabidiol (delegate initiated)
4 Interim decisions on proposed amendments referred to the Advisory Committee on Medicines and Chemicals Scheduling in joint session (Joint ACMS-ACCS #25, June 2020) Note New text is shown as green, larger font, with a horizontal line above it.
 www.tga.gov.au
www.tga.gov.au
So, in review, an interim decision has already approved down scheduling of some CBD products, from 4 to 3, (schedule 4 needs prescription, schedule 3 is Over The Counter (OTC) based on adherence to certain changes such as strength, packaging, advertising etc...............basically dotting i"s and crossing T's sort of stuff.
There also has been recent testing of goods being imported (accessed currently under the SAS scheme), the results looked favorable to the importers also with most products falling within test & approval guidelines, being strength, contaminates etc. A couple fell outside the strength ranges, notably one slightly below and one slightly above. No biggies.
The decision will mean products will start being registered in Australia for over the counter (OTC) sale in Australian Pharmacies for certain "low dose" CBD (cannabidiol) products which may be of benefit for those with insomnia, anxiety, pain, inflammation, epilepsy seizures etc.
Implementation of final decision is scheduled for 1/7/2021 as per the link provided above from TGA, meaning goods could be prepared, registered and available for sale on that date.
Further, the EU has opened up access by the following declaration, which in my opinion, is a very belated decision.

I have already purchased CPH, EXL, AC8.
Others considered, ZLD, MDC, CAN.
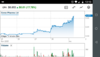
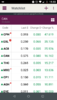
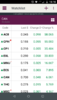
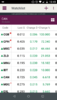
I note CPH, ZLD & MDC are certainly moving in the right direction today.
Cheers
26 November 2020
Update on TGA reclassification of CBD
▪ TGA decision regarding amendments to down schedule cannabidiol (CBD) products to schedule 3 medicine expected in late December 2020 (i)
▪ Schedule amendment would allow Australian consumers to purchase CBD products following consultation with a pharmacist without the need for a prescription
▪ Unlocks a large market opportunity for Creso Pharma – Australian market estimated to be worth upwards of A$200m (ii)
▪ Creso remains well funded to capitalise in Australian market following final decision.
For anyone who wonders why I think the ASX CPH announcement is relevant in the bigger picture and my opinion of why "it will be approved", I would encourage you to digest the information found here... (a wee bit of light reading)

4.1 Cannabidiol (private application) and cannabidiol (delegate initiated)
4 Interim decisions on proposed amendments referred to the Advisory Committee on Medicines and Chemicals Scheduling in joint session (Joint ACMS-ACCS #25, June 2020) Note New text is shown as green, larger font, with a horizontal line above it.
So, in review, an interim decision has already approved down scheduling of some CBD products, from 4 to 3, (schedule 4 needs prescription, schedule 3 is Over The Counter (OTC) based on adherence to certain changes such as strength, packaging, advertising etc...............basically dotting i"s and crossing T's sort of stuff.
There also has been recent testing of goods being imported (accessed currently under the SAS scheme), the results looked favorable to the importers also with most products falling within test & approval guidelines, being strength, contaminates etc. A couple fell outside the strength ranges, notably one slightly below and one slightly above. No biggies.
The decision will mean products will start being registered in Australia for over the counter (OTC) sale in Australian Pharmacies for certain "low dose" CBD (cannabidiol) products which may be of benefit for those with insomnia, anxiety, pain, inflammation, epilepsy seizures etc.
Implementation of final decision is scheduled for 1/7/2021 as per the link provided above from TGA, meaning goods could be prepared, registered and available for sale on that date.
Further, the EU has opened up access by the following declaration, which in my opinion, is a very belated decision.
I have already purchased CPH, EXL, AC8.
Others considered, ZLD, MDC, CAN.
I note CPH, ZLD & MDC are certainly moving in the right direction today.
Cheers