Sean K
Moderator
- Joined
- 21 April 2006
- Posts
- 22,017
- Reactions
- 11,000
Circadian provides management and funding for the development and commercialisation of Australian biomedical research. It has major holdings in Optiscan, Metabolic Pharmaceuticals, and Antisence amongst others. It also maintains an active research and development program.
For those interested in getting into a biotech, this offers a potentially lower risk (multi project) entry.
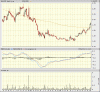
Has come off recent lows and been generally heading up for the past few months, forming a giant cup. Looks set to reach $2.00 - the next level of resistance.
With biotech becomming more the flavour of the month recently, might see more attention on this.
Will add in some more detail on their investments soon.
www.circadian.com.au
(not holding)
(I did have Optiscan a while ago)
For those interested in getting into a biotech, this offers a potentially lower risk (multi project) entry.
Has come off recent lows and been generally heading up for the past few months, forming a giant cup. Looks set to reach $2.00 - the next level of resistance.
With biotech becomming more the flavour of the month recently, might see more attention on this.
Will add in some more detail on their investments soon.
www.circadian.com.au
(not holding)
(I did have Optiscan a while ago)